How is pain mediated? How does injury cause pain?
Injury causes (even a bacterial infection causes an injury to a cell / cell membrane) disruption of cell membrane. What is the cell membrane made of?
Cells – Cells have cell membranes which are made of phospholipid bilayer.
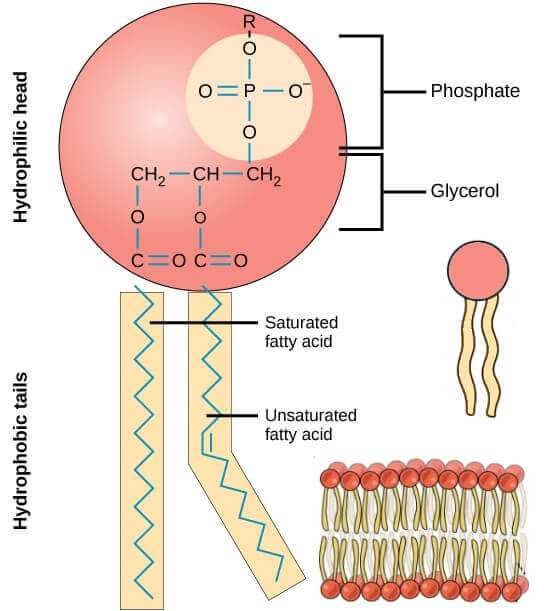
What is a phospholipid?
It is basically a glycerol molecule (glycerol has 3 carbon chain) with 2 fatty acid chains and one phosphate molecule. The fatty acid chains are hydrophobic (fats do not mix with water) whereas the phosphate head is hydrophilic (it mixes easily with water). The arrangement of phosphate, glycerol and the fatty acid chains give the phospholipid its shape. See image below.
What happens with cell injury?
When cell membranes are injured (which are mainly phospholipid) they activate an enzyme called ‘phospholipase’. This enzyme converts some of the phospholipids into prostaglandins. These prostaglandins mediate pain, inflammation and fever.
What are Prostaglandins?
Prostaglandins are a type of eicosanoids. What are eicosanoids?
Eicosanoids
Eicosanoids are paracrine hormones. Paracrine hormones are those which act close to the site of synthesis rather than travelling via blood to distant sites. These are fatty acid derivatives. All eicosanoids are derived from arachidonic acid which is a 20 carbon polyunsaturated fatty acid. This is where they take their general name from. Greek “Eikosi” = twenty.
There are three classes of eicosanoids:
- Prostaglandins (PG)
- Thromboxanes
- Leukotrienes
Prostaglandins (PG) were first discovered in ‘prostate glands’ (by Swedish scientists), so they are called ‘prostaglandins’. They were originally grouped into 2 types PGE (ether soluble) & PGF (fosfat soluble; fosfat is swedish for phosphate; which means buffer soluble). There are various subtypes PGE1, 2 PGF1 etc. They have various functions.
Thromboxanes are produced by platelets and play a role in clotting.
Nonsteroidal anti inflammatory drugs (NSAIDs) such as aspirin and ibuprofen block the formation of prostaglandins and thromboxanes from arachidonate by inhibiting the enzyme cyclooxygenase (prostaglandin H2 synthase).
Leukotrienes were first found in leucocytes. They are mediators of inflammation. They contain 3 conjugated double bonds (hence their name “triene”. In hydrocarbons, double bonds are signified by suffix “ene”. so 3 double bonds make it ‘tri ene‘). These induce contraction of the smooth muscle lining the airways to the lung which causes bronchoconstriction. They also cause smooth muscle contraction of intestine and uterus. Overproduction of leukotrienes causes asthmatic attacks. Leukotriene synthesis is one target of antiasthmatic drugs such as prednisolone. Mast cells and eosinophils release leukotrienes. They are mediators of anaphylaxis.
Arachidonic Acid Pathway
Arachidonic acid is present in cell membranes and accounts for 5 to 15% of the fatty acids in phospholipids. After arachidonic acid is released from phospholipids by the action of phospholipase A2, the cyclooxygenase (COX) and peroxidase activities of COX (also called prostaglandin H2 synthase) catalyze the production of PGH2, the precursor of other prostaglandins and thromboxanes.
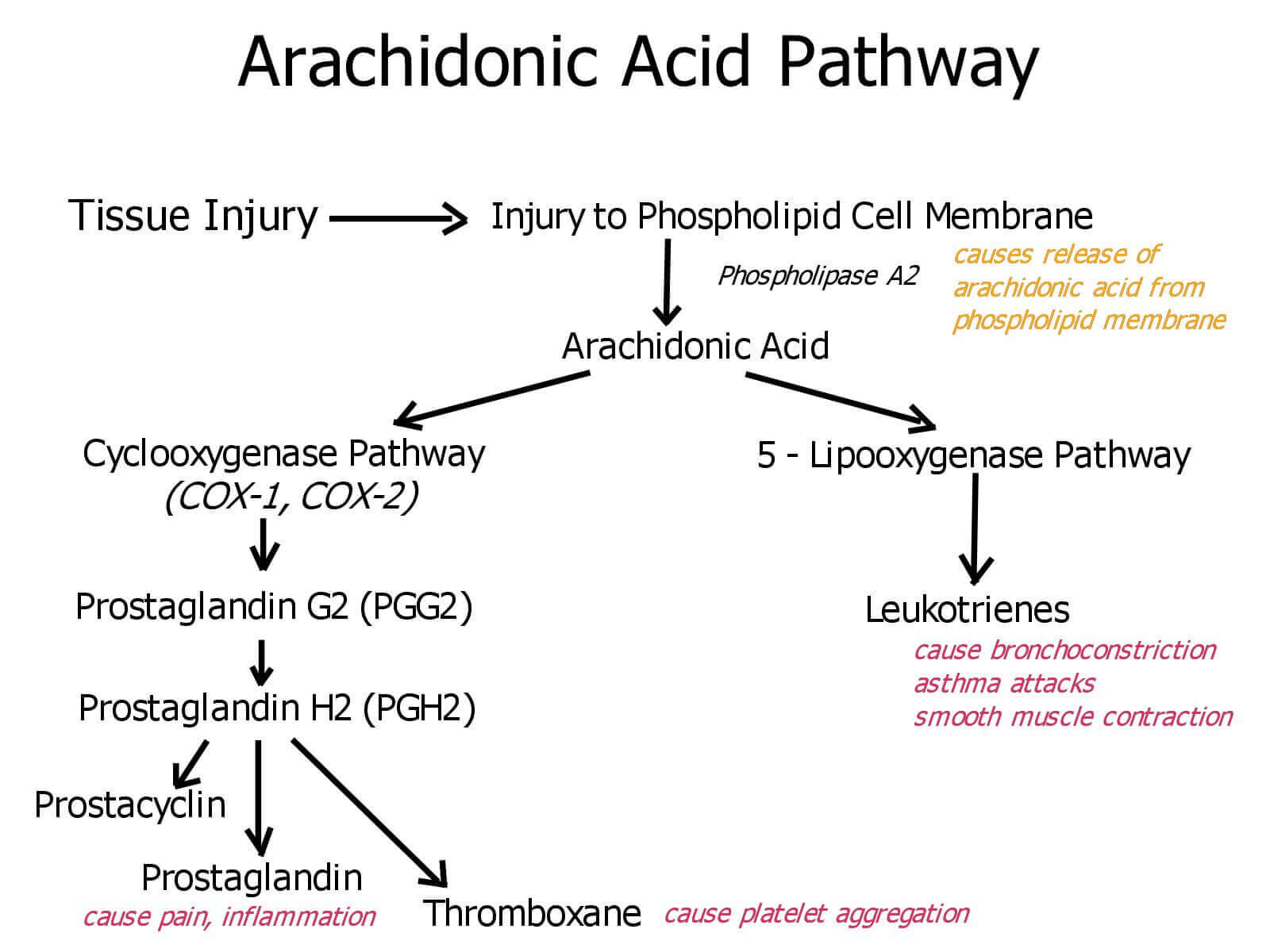
Mammals have two isozymes of prostaglandin H2 synthase, COX-1 and COX-2. COX-1 is responsible for the synthesis of the prostaglandins that regulate the secretion of gastric mucin, and COX-2 for the prostaglandins that mediate inflammation, pain, and fever.
Pain can be relieved by inhibiting COX-2. The first drug widely marketed for this purpose was aspirin.
Aspirin irreversibly inactivates the cyclooxygenase activity of both COX
isozymes. The synthesis of prostaglandins and thromboxanes is thereby inhibited. Ibuprofen also inhibits the same pair of enzymes.
However, the inhibition of COX-1 can result in undesired side effects including stomach irritation and more serious conditions.
Selective COX-2 inhibitors were therefore prepared to counter severe pain such as Rofecoxib and Celecoxib but later studies showed an increased risk of heart attacks and stroke with the use of these drugs. Therefore they were withdrawn. Celecoxib is still used with caution.