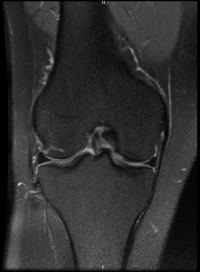
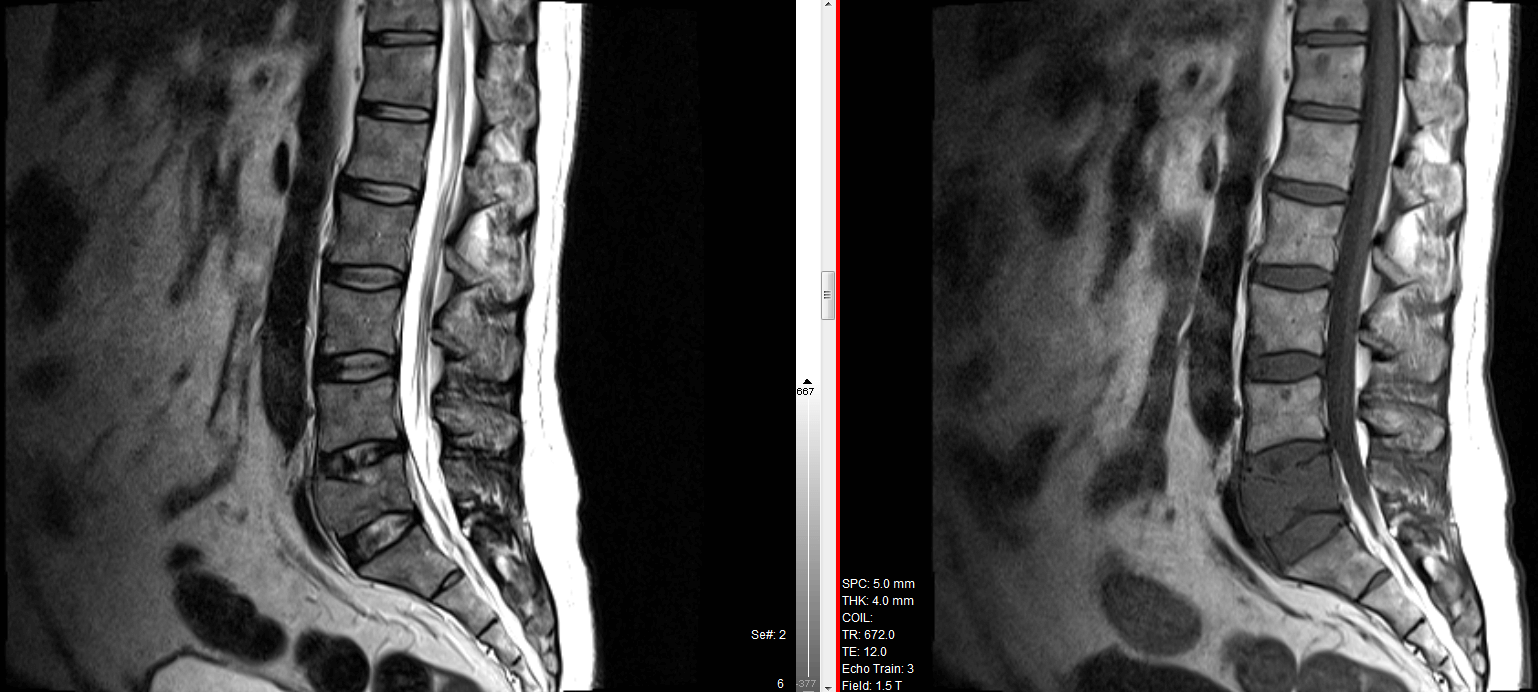
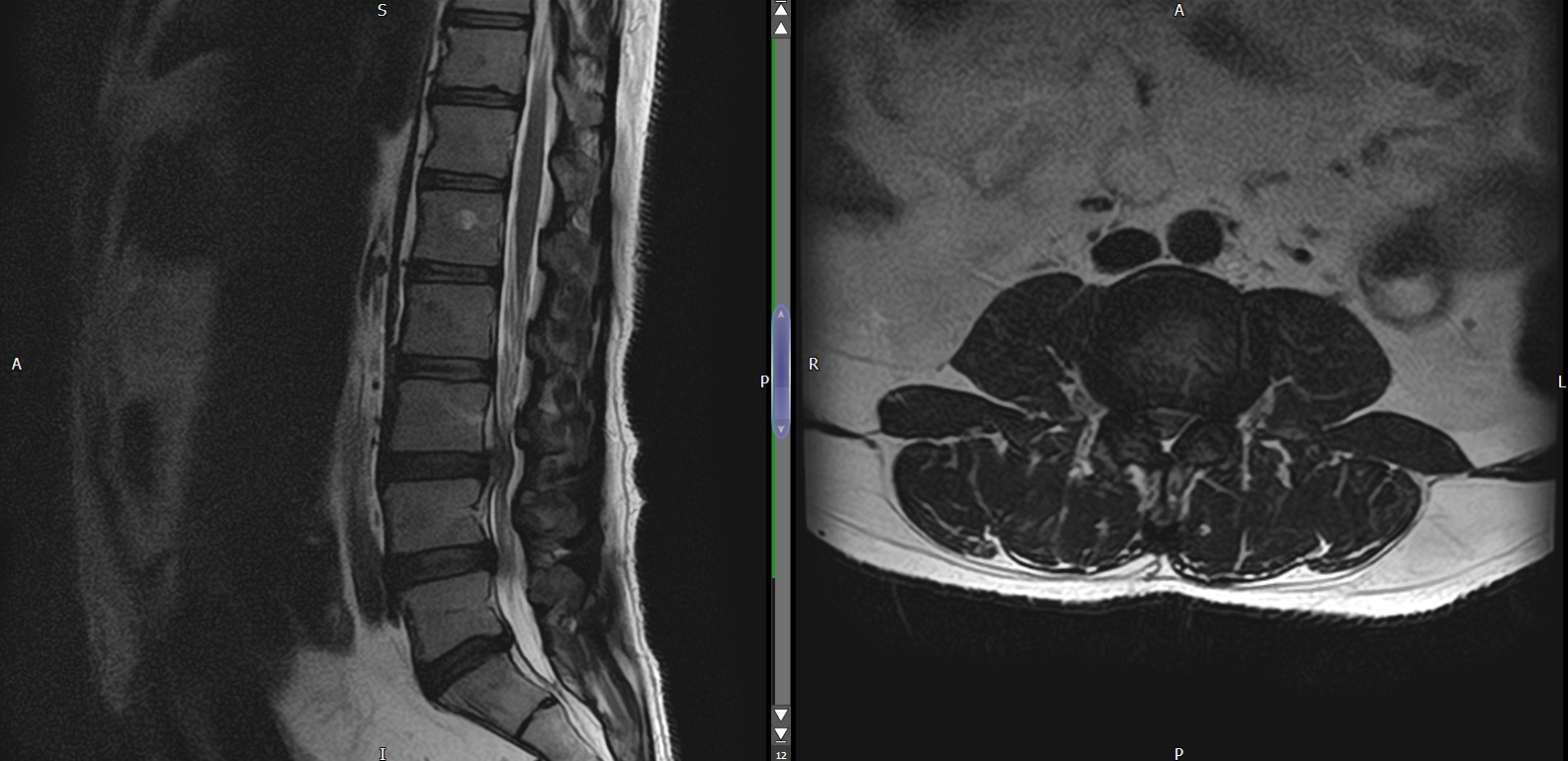
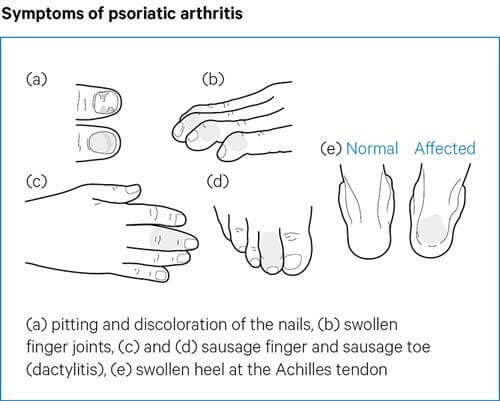
Mole - It is a unit of measurement to count small numbers.
- Abbreviation for Mole is mol
- 1 mol = 6.02214076×1023 particles. The "particles" could be something small, like electrons or atoms, or something large, like chairs or cars.
Read more on moles
Atomic Number
The number of protons in an atom is called the atomic number. e.g. carbon has 6 protons and therefore its atomic number is six.
Number of neutrons for a given element can vary. Forms of the same atom that differ only in their number of neutrons are called isotopes.
Mass Number
Number of neutrons and protons in an element is its mass number.
- mass number = protons + neutrons
Atomic Mass
Atomic mass is the sum of the masses of the protons, neutrons, and electrons in an atom. However, electrons have so much less mass than protons and neutrons that they don't factor into the calculation. So, the atomic mass is the sum of the masses of protons and neutrons.
- The atomic mass of a single atom is simply its total mass and is expressed in atomic mass units or amu.
- an atom of carbon with six neutrons i.e. carbon-12, has an atomic mass of 12 amu.