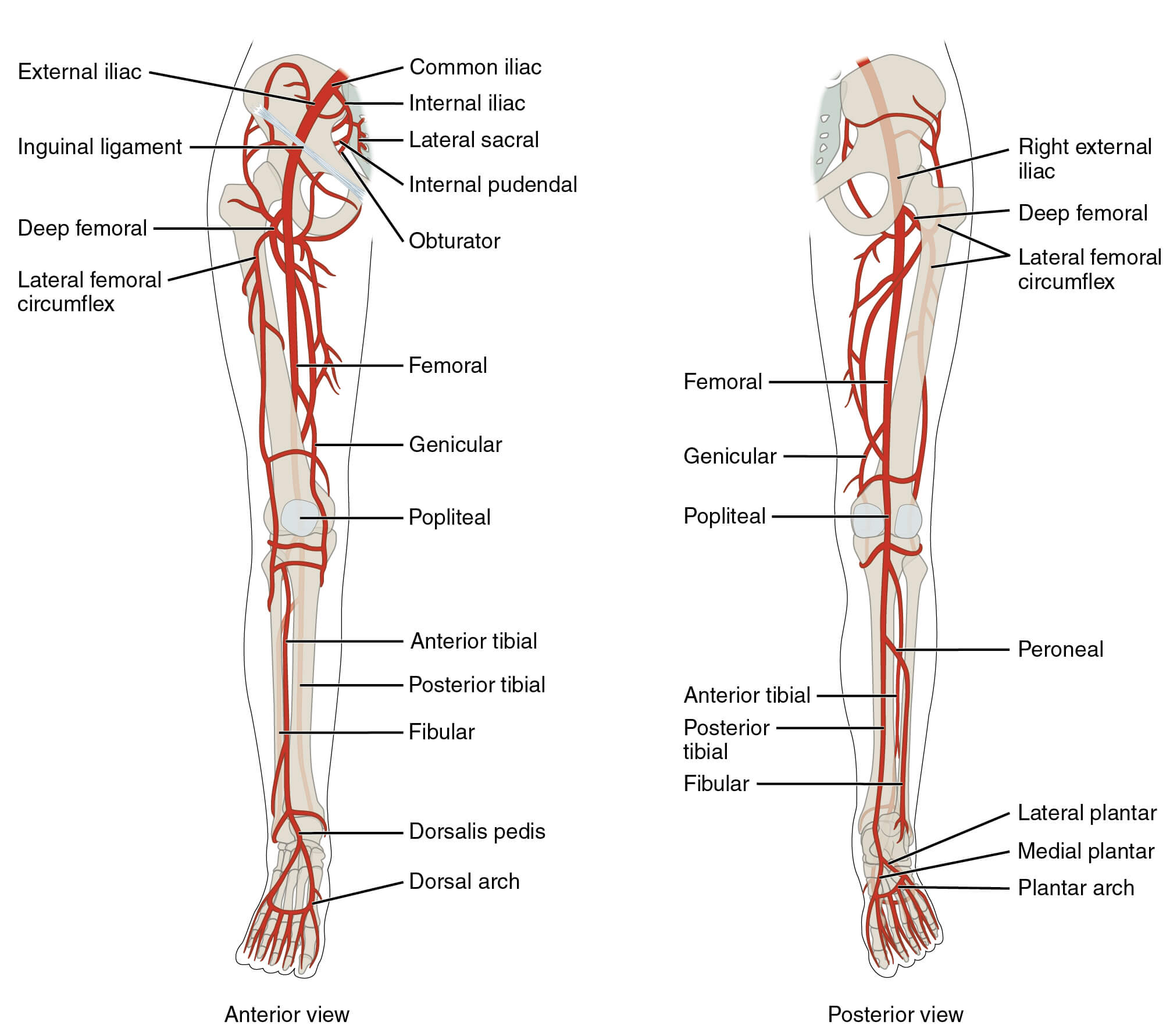
What is the overall risk of causing a bleed into a joint with intra-articular injections in patients on NOAC/DOAC
Various studies have reported their results on use of intra-articular injections in patients taking blood thinners. Some of them are listed below. Studies related to other procedures like transforaminal injections and dental extractions are also listed as they too are interventional procedures with a risk of causing a bleed in patients on anticoagulants.
Overall the impression is that intra-articular injections are deemed safe with NOAC/DOAC and do not usually require them to be discontinued prior to carrying out the procedure.
The Risk of Bleeding Complications in Intra-Articular Injections and Arthrocentesis in Patients on Novel Oral Anticoagulants: A Systematic Review - Tarar & Khan 2021
- Four studies were deemed suitable for the systematic review analysis.
- Total of 668 patients had injections/aspiration procedures while they were on three different novel oral anticoagulants namely Rivaroxaban, Apixaban, and Dabigatran.
- Only one patient joint had a bleeding complication and the patient was on Dabigatran.
Conclusion: The results of this systematic review show that it is relatively safe to perform joint injections and arthrocentesis whilst continuing on Novel oral anticoagulation.
Arthrocentesis and Joint Injection in Patients Receiving Direct Oral Anticoagulants – Yui et al – Mayo Clinic 2017
- DOAC (direct-acting anticoagulants) or NOAC (Novel Oral Anticoagulants) – rivaroxaban [Xarelto], apixaban [Eliquis], and dabigatran [Pradaxa]
- Joint aspiration / injection seems safe in patients on DOAC.
- In 1050 consecutive procedures, there were no bleeding complications.
Conclusion – Arthrocentesis and joint injections in patients receiving DOAC therapy are safe procedures, and there is no need to withhold anticoagulation treatment before the procedure.
The Risks of Continuing or Discontinuing Anticoagulants for Patients Undergoing Common Interventional Pain Procedures – Endres et al, Pain Medicine 2017
- Aim of the study was to determine if continuing or discontinuing anticoagulants for pain procedures was associated with a detectable risk of complications.
- This was an observational study where some clinicians continued to do interventions without stopping anticoagulants whilst some discontinued anticoagulants.
- There were no complications attributable to anticoagulants encountered in 4,766 procedures in which anticoagulants were continued.
- In 2,296 procedures in which anticoagulants were discontinued, nine patients suffered serious morbidity, including two deaths.
Conclusion: Lumbar transforaminal injections, lumbar medial branch blocks, trigger point injections, and sacroiliac joint blocks appear to be safe in patients who continue anticoagulants. In patients who discontinue anticoagulants, although low (0.2%) the risk of serious complications is not zero, and must be considered when deciding between continuing and discontinuing anticoagulants
Update of a Study of Not Ceasing Anticoagulants for Patients Undergoing Injection Procedures for Spinal Pain – Endres et al, Pain Medicine 2020
- A total of 1,936 consecutive patients were prospectively monitored during a total of 12,723 injection procedures.
- The prevalence of hemorrhagic complications was tallied for a variety of procedures performed on patients who ceased or continued various anticoagulants.
- No hemorrhagic complications occurred in any patient who continued anticoagulants.
Conclusion: Lumbar transforaminal injections and lumbar facet injections have a very low rate of hemorrhagic complications when patients continue to take anticoagulants
Anticoagulant and Antiplatelet Management for Spinal Procedures: A Prospective, Descriptive Study and Interpretation of Guidelines - Goodman et al, Pain Medicine 2017
- Epidural hematoma is a rare complication of spinal procedures.
- 4,253 injection sites. 197 (4.6%) were performed in 74 patients on antiplatelet/anticoagulants.
- No clinically evident bleeding events were observed in patients on antiplatelet/anticoagulant medications for lumbar transforaminal epidural (N = 90), posterior-approach facet joint (N = 62), lumbar intradiscal (N = 11), lumbar sympathetic (N = 3), and sacroiliac (N = 5) injections or in radiofrequency neurotomy procedures (N = 26).
- One patient suffered epidural haematoma but the patient was not on anticoagulants.
Conclusions: Continuing antiplatelet and anticoagulant medications for intermediate- to low-risk interventional spine procedures may be advisable.
Risks and Benefits of Ceasing or Continuing Anticoagulant Medication for Image-Guided Procedures for Spine Pain: A Systematic Review - Smith et al, Pain Medicine 2018
- Out of 120 studies, 14 were included in review.
- Procedures involving interlaminar access carry a nonzero risk of hemorrhagic complications, regardless of whether anticoagulants are ceased or continued.
- Hemorrhagic complications have not been reported in other spinal procedures, and case series indicate that they are safe when performed in patients who continue anticoagulants.
- Three articles reported the adverse effects of ceasing anticoagulants, with serious consequences, including death.
Conclusion: Other than for interlaminar procedures, the evidence does not support the view that anticoagulant and antiplatelet medication must be ceased before image-guided spine pain procedures. Meanwhile, the evidence shows that ceasing anticoagulants carries a risk of serious consequences, including death.
Determination of a safe INR for joint injections in patients taking warfarin – Bashir et al – Annals Royal College of Surgeons of England 2015
- 41 patients on warfarin were given 86 injections either landmark guided by ortho surgeons or US guided by radiologists. None had any haemarthrosis post injection.
Conclusion – With a mean INR of 2.77 (range, 1.7-5.5) and a maximum INR within this group of 5.5, joint injections to the shoulder and knee can be undertaken safely in primary or secondary care settings despite the patient taking warfarin
Safety of joint and soft tissue injections in patients on warfarin anticoagulation – Conway et al – Rheumatology 2013
- 2 Groups with 32 procedures each. For one group warfarin was stopped, other group continued to take warfarin.
- In group who continued on warfarin, 27 were joint injections and 5 were soft tissue injections.
- There were no clinical hemarthroses or complications in either group.
Conclusion – Joint and soft tissue injections appear to be safe in patients receiving warfarin anticoagulation with an INR <3
Hand Corticosteroid Injections in Patients on “Blood Thinners” – Malige & Matullo – Hand NY 2020
- There were 6 complications after 433 injections (1.6%) placed in patients on blood thinners and 6 complications after 1040 injections (0.6%) placed in patients not on blood thinners.
- Conclusion – With the complication rate of corticosteroid injections being so low, even in patients taking “blood thinners,” the fear of adverse reactions should not preclude a physician from using this treatment modality to prevent surgical intervention
Dental extractions on direct oral anticoagulants vs. warfarin: The DENTST study – Research and practice in thrombosis and haemostasis – Brennan et al 2020
Conclusion – Dental extractions on patients continuing DOACs led to bleeding rates similar to patients on warfarin with an INR between 2.0 and 4.0. There is no need to adjust DOAC dosing prior to dental extractions.
Should we fear direct oral anticoagulants more than vitamin K antagonists in simple single tooth extraction? A prospective comparative study – Clinical oral investigations – Berton et al 2019
Clinical relevance: Patients assuming DOACs can be treated similarly to patients in VKAs therapy with INR index between 2 and 3. Non-ceasing of DOAC therapy seems to be appropriate for simple single dental extractions.
What is Half Life | Drugs
Half life is the time it takes in the body for the concentration of the drug to reduce by half.
For example, if a 100 mg of a drug has a half life of 1 hour or 60 minutes then the following estimates can be made.
- After 1 hour or 60 minutes of administration of the drug, 50mg will remain in the body.
- After 2 hours, 25mg will remain.
- After 3 hours, 12.5mg will remain.
- so on and so forth.
It is considered that most drugs have negligible effect after 4-5 half life cycles.
It is not that straightforward however. Drug metabolism depends upon various body organs such as liver and kidneys. Someone with kidney dysfunction for example may have longer drug half lives as it will take longer for the drug to leave the system as the function of kidneys is impaired. Likewise for drugs metabolised in liver, it may take longer in someone with liver disease.
Half life of Apixaban – Eliquis
- Half life of Apixaban is around 12 hours
Half life of Rivaroxaban – Xarelto
- Elimination of rivaroxaban from plasma occurs with a terminal half-life of 5–9 h in healthy young subjects and 11–13 h in elderly subjects
Half life of Dabigatran – Pradaxa
- In patients with normal renal function, approximately 80% of an intravenous dabigatran dose is excreted in urine with an elimination half-life of 12–17 h